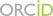Chromatin and cell biology

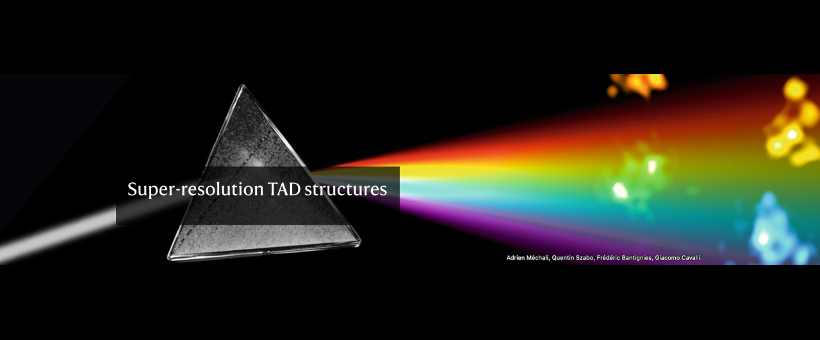
The genome is more than a linear string of genes. It has secondary, tertiary and quaternary higher-order structures. Strings of genes organize in chromosomal domains that are characterized by one of relatively few different types of chromatin, either repressive or activating. In the 3D space, each of the elements in one domain makes frequent contacts with other elements in the same domain. These entities have been called physical- or topologically associated- domains (TADs).
At a chromosome level, individual TADs form contacts with other TADs, preferentially of the same type, in order to build ordered 3D architectures that are called chromosome territories. Finally, different chromosomes organize non randomly in the nuclear space.
Therefore, the eukaryotic genome is highly organized in 3D and this regulation can be transmitted or modulated during the life of cells and organisms.
This sophisticated chromosomal choreography involves thousands of different players – DNA sequences, RNAs and proteins – but rather than combining in infinite numbers of ways, these components organize a relatively limited number of types of chromatin, either active or repressive. In particular, two main groups of genome regulatory components are proteins of the Polycomb Group (PcG) and of the trithorax Group (trxG). PcG proteins are maintain the memory of silent states of gene expression through cell physiology and multiple cell divisions, while trxG members maintain active chromatin states
These proteins are able to recognize regulatory states of their target genes and to maintain these states even after disappearance of the primary transcriptional regulators that have induced them in the first place. Remarkably, these states can also be transmitted to a fraction of the progeny over multiple generations. In our lab, we aim at understanding the principle governing 3D genome organization, its functional implications, and the molecular mechanisms by which PcG and trxG proteins regulate their target genes, convey inheritance of chromatin states and orchestrate development.
To reach this goal, we employ a variety of complementary approaches and techniques in the areas of molecular, cellular and developmental biology, genomics and bioinformatics.
Publications of the team
Phase separation and inheritance of repressive chromatin domains.
Akilli N, Cheutin T, Cavalli G
Drosophila TET acts with PRC1 to activate gene expression independently of its catalytic activity.
Gilbert G, Renaud Y, Teste C, Anglaret N, Bertrand R, Hoehn S, Jurkowski TP, Schuettengruber B, Cavalli G, Waltzer L, Vandel L

Transient loss of Polycomb components induces an epigenetic cancer fate.
Parreno V, Loubiere V, Schuettengruber B, Fritsch L, Rawal CC, Erokhin M, Győrffy B, Normanno D, Di Stefano M, Moreaux J, Butova NL, Chiolo I, Chetverina D, Martinez AM, Cavalli G
Epigenetic inheritance in adaptive evolution.
Sabarís G, Fitz-James MH, Cavalli G
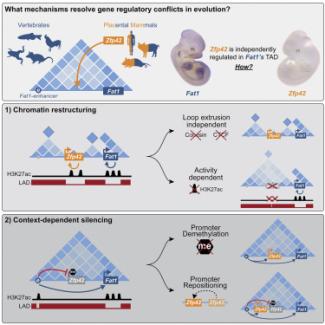
Repression and 3D-restructuring resolves regulatory conflicts in evolutionarily rearranged genomes.
Ringel AR, Szabo Q, Chiariello AM, Chudzik K, Schöpflin R, Rothe P, Mattei AL, Zehnder T, Harnett D, Laupert V, Bianco S, Hetzel S, Glaser J, Phan MHQ, Schindler M, Ibrahim DM, Paliou C, Esposito A, Prada-Medina CA, Haas SA, Giere P, Vingron M, Wittler L, Meissner A, Nicodemi M, Cavalli G, Bantignies F, Mundlos S, Robson MI
A shared ancient enhancer element differentially regulates the bric-a-brac tandem gene duplicates in the developing Drosophila leg.
Bourbon HG, Benetah MH, Guillou E, Mojica-Vazquez LH, Baanannou A, Bernat-Fabre S, Loubiere V, Bantignies F, Cavalli G, Boube M
Comprehensive characterization of the epigenetic landscape in Multiple Myeloma.
Alaterre E, Ovejero S, Herviou L, de Boussac H, Papadopoulos G, Kulis M, Boireau S, Robert N, Requirand G, Bruyer A, Cartron G, Vincent L, Martinez AM, Martin-Subero JI, Cavalli G, Moreaux J
HiCmapTools: a tool to access HiC contact maps.
Chang JM, Weng YF, Chang WT, Lin FA, Cavalli G
SETDB1/NSD-dependent H3K9me3/H3K36me3 dual heterochromatin maintains gene expression profiles by bookmarking poised enhancers.
Barral A, Pozo G, Ducrot L, Papadopoulos GL, Sauzet S, Oldfield AJ, Cavalli G, Déjardin J
Mechanisms of Polycomb group protein function in cancer.
Parreno V, Martinez AM, Cavalli G
Molecular mechanisms of transgenerational epigenetic inheritance.
Fitz-James MH, Cavalli G
Clinical Correlations of Polycomb Repressive Complex 2 in Different Tumor Types.
Erokhin M, Chetverina O, Győrffy B, Tatarskiy VV, Mogila V, Shtil AA, Roninson IB, Moreaux J, Georgiev P, Cavalli G, Chetverina D
Understanding 3D genome organization by multidisciplinary methods
Ivana Jerkovic´ & Giacomo Cavalli
test
test1, test2
Regulation of single-cell genome organization into TADs and chromatin nanodomains
Szabo Q., Donjon A., Jerkovic I., Papadopoulos G.L., Cheutin T., Bonev B., Nora E., Bruneau B.G., Bantignies F., Cavalli, G.
4D Genome Rewiring during Oncogene-Induced and Replicative Senescence
Sati S., Bonev, B., Szabo, Q., Jost, D., Bensadoun, P., Serra, F., Loubiere
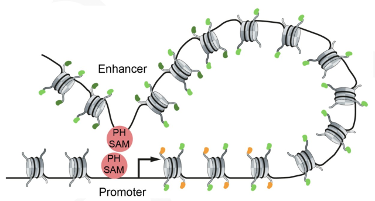
Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping.
Loubiere V, Papadopoulos GL, Szabo Q, Martinez AM, Cavalli G

Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping.
Loubiere V, Papadopoulos GL, Szabo Q, Martinez AM, Cavalli G
Global chromatin conformation differences in the Drosophila dosage compensated chromosome X.
Pal K, Forcato M, Jost D, Sexton T, Vaillant C, Salviato E, Mazza EMC, Lugli E, Cavalli G, Ferrari F
Global chromatin conformation differences in the Drosophila dosage compensated chromosome X.
Pal K, Forcato M, Jost D, Sexton T, Vaillant C, Salviato E, Mazza EMC, Lugli E, Cavalli G, Ferrari F
The multiscale effects of polycomb mechanisms on 3D chromatin folding.
Cheutin T, Cavalli G
The multiscale effects of polycomb mechanisms on 3D chromatin folding.
Cheutin T, Cavalli G
Higher-Order Chromosomal Structures Mediate Genome Function.
Jerković I, Szabo Q, Bantignies F, Cavalli G
Higher-Order Chromosomal Structures Mediate Genome Function.
Jerković I, Szabo Q, Bantignies F, Cavalli G

Advances in epigenetics link genetics to the environment and disease
Giacomo Cavalli and Edith Heard

Advances in epigenetics link genetics to the environment and disease
Giacomo Cavalli and Edith Heard
Principles of genome folding into topologically associating domains.
Szabo Q, Bantignies F, Cavalli G
Microscopy-Based Chromosome Conformation Capture Enables Simultaneous Visualization of Genome Organization and Transcription in Intact Organisms.
Cardozo Gizzi AM, Cattoni DI, Fiche JB, Espinola SM, Gurgo J, Messina O, Houbron C, Ogiyama Y, Papadopoulos GL, Cavalli G, Lagha M, Nollmann M
Cell Fate and Developmental Regulation Dynamics by Polycomb Proteins and 3D Genome Architecture.
Loubiere V, Martinez AM, Cavalli G
EZH2 is overexpressed in transitional preplasmablasts and is involved in human plasma cell differentiation.
Herviou L, Jourdan M, Martinez AM, Cavalli G, Moreaux J
+
Maintenance of genome integrity during DNA replication
PRC2 targeting is a therapeutic strategy for EZ score defined high-risk multiple myeloma patients and overcome resistance to IMiDs.
Herviou L, Kassambara A, Boireau S, Robert N, Requirand G, Müller-Tidow C, Vincent L, Seckinger A, Goldschmidt H, Cartron G, Hose D, Cavalli G, Moreaux J
+
Maintenance of genome integrity during DNA replication
Challenges and guidelines toward 4D nucleome data and model standards.
Marti-Renom MA, Almouzni G, Bickmore WA, Bystricky K, Cavalli G, Fraser P, Gasser SM, Giorgetti L, Heard E, Nicodemi M, Nollmann M, Orozco M, Pombo A, Torres-Padilla ME
Loss of PRC1 induces higher-order opening of Hox loci independently of transcription during Drosophila embryogenesis.
Cheutin T, Cavalli G
[EZH2 is therapeutic target for personalized treatment in multiple myeloma].
Herviou L, Cavalli G, Moreaux J
+
Maintenance of genome integrity during DNA replication
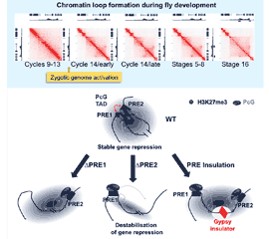
Polycomb-Dependent Chromatin Looping Contributes to Gene Silencing during Drosophila Development.
Ogiyama Y, Schuettengruber B, Papadopoulos GL, Chang JM, Cavalli G
TADs are 3D structural units of higher-order chromosome organization in Drosophila.
Szabo Q, Jost D, Chang JM, Cattoni DI, Papadopoulos GL, Bonev B, Sexton T, Gurgo J, Jacquier C, Nollmann M, Bantignies F, Cavalli G
Technical Review: A Hitchhiker's Guide to Chromosome Conformation Capture.
Grob S, Cavalli G
Multi-scale 3D genome rewiring during mouse neural development.
Bonev, B., Mendelson Cohen, N., Szabo, Q., Fritsch, L., Papadopoulos, G., Lubling, Y., Xu, X., Hugnot, JP., Tanay, A., Cavalli, G.
Genome Regulation by Polycomb and Trithorax: 70 Years and Counting
Schuettengruber, B., Bourbon, HM., Di Croce, L., Cavalli, G.
Chromosome topology guides the Drosophila Dosage Compensation Complex for target gene activation
Schauer T, Ghavi-Helm Y, Sexton T, Albig C, Regnard C, Cavalli G, Furlong EE, Becker PB.
Stable Polycomb-dependent transgenerational inheritance of chromatin states in Drosophila
Ciabrelli F, Comoglio F, Fellous S, Bonev B, Ninova M, Szabo Q, Xuéreb A, Klopp C, Aravin A, Paro R, Bantignies F, Cavalli G
Three-Dimensional Genome Organization and Function in Drosophila
Schwartz YB, Cavalli G.
Chromosome conformation capture technologies and their impact in understanding genome function
Sati S, Cavalli G.
Single-cell absolute contact probability detection reveals chromosomes are organized by multiple low-frequency yet specific interactions
Cattoni DI, Gizzi AMC, Georgieva M, Di Stefano M, Valeri A, Chamousset D, Houbron C, Déjardin S, Fiche JB, González I, Chang JM, Sexton T, Marti-Renom MA, Bantignies F, Cavalli G, Nollmann M.
Organization and function of the 3D genome
Bonev B, Cavalli G.
Chromosome Conformation Capture on Chip (4C): Data Processing
Leblanc B, Comet I, Bantignies F, Cavalli G.
Following the Motion of Polycomb Bodies in Living Drosophila Embryos
Cheutin T, Cavalli G.
Coordinate redeployment of PRC1 proteins suppresses tumor formation during Drosophila development
Loubière, V., Delest, A., Thomas, A., Bonev, B., Schuettengruber, B., Sati, S., Martinez AM., Cavalli, G.
Regulation of Genome Architecture and Function by Polycomb Proteins
Entrevan M, Schuettengruber B, Cavalli G
EZH2 in normal hematopoiesis and hematological malignancies
Herviou L, Cavalli G, Cartron G, Klein B, Moreaux J.
PRC1 proteins orchestrate three-dimensional genome architecture
Cavalli, G.
Enhancer of zeste acts as a major developmental regulator of Ciona intestinalis embryogenesis
Le Goff E, Martinand-Mari C, Martin M, Feuillard J, Boublik Y, Godefroy N, Mangeat P, Baghdiguian S, Cavalli G
Distinct polymer physics principles govern chromatin dynamics in mouse and Drosophila topological domains
Ea V, Sexton T, Gostan T, Herviou L, Baudement MO, Zhang Y, Berlivet S, Le Lay-Taha MN, Cathala G, Lesne A, Victor JM, Fan Y, Cavalli G, Forné T.
Histone H3 Serine 28 Is Essential for Efficient Polycomb-Mediated Gene Repression in Drosophila
Yung PY, Stuetzer A, Fischle W, Martinez AM, Cavalli G
The Role of Chromosome Domains in Shaping the Functional Genome
Sexton, T., Cavalli, G.
Developmental determinants in non-communicable chronic diseases and ageing
Bousquet J, Anto JM, Berkouk K, Gergen P, Pinto Antunes J, Augé P, Camuzat T, Bringer J, Mercier J, Best N, Bourret R, Akdis M, Arshad SH, Bedbrook A, Berr C, Bush A, Cavalli G, Charles MA, Clavel-Chapelon F, Gillman M, Gold DR, Goldberg M, Holloway JW, Iozzo P, Jacquemin S, Jeandel C, Kauffmann F, Keil T, Koppelman GH, Krauss-Etschmann S, Kuh D, Lehmann S, Lodrup Carlsen KC, Maier D, Méchali M, Melén E, Moatti JP, Momas I, Nérin P, Postma DS, Ritchie K, Robine JM, Samolinski B, Siroux V, Slagboom PE, Smit HA, Sunyer J, Valenta R, Van de Perre P, Verdier JM, Vrijheid M, Wickman M, Yiallouros P, Zins M.
Chromatin driven behavior of topologically associating domains
Ciabrelli F, Cavalli G.
Cooperativity, Specificity, and Evolutionary Stability of Polycomb Targeting in Drosophila
Schuettengruber B, Oded Elkayam N, Sexton T, Entrevan M, Stern S, Thomas A, Yaffe E, Parrinello H, Tanay A, Cavalli G.
Topological organization of Drosophila hox genes using DNA fluorescent in situ hybridization
Bantignies, F., Cavalli, G.
Modeling epigenome folding: formation and dynamics of topologically associated chromatin domains
Jost D, Carrivain P, Cavalli G, Vaillant C.
Identification of Regulators of the Three-Dimensional Polycomb Organization by a Microscopy-Based Genome-wide RNAi Screen
Gonzalez I, Mateos-Langerak J, Thomas A, Cheutin T, Cavalli G.
A RING to Rule Them All: RING1 as Silencer and Activator
Cavalli, G.
Polycomb silencing: from linear chromatin domains to 3D chromosome folding
Cheutin T, Cavalli G
Chromosomes: now in 3D!
Cavalli, G.
Three-dimensional genome organization: a lesson from the Polycomb-Group proteins
Bantignies, F.
PRC2 Controls Drosophila Oocyte Cell Fate by Repressing Cell Cycle Genes.
Iovino N, Ciabrelli F, Cavalli G.
The 3D Genome Shapes Up For Pluripotency.
Sexton T, Cavalli G.
Functional implications of genome topology
Cavalli, G., Mistelli, T.
Polycomb domain formation depends on short and long distance regulatory cues.
Schuettengruber B, Cavalli G.
Chromosomal domains: epigenetic contexts and functional implications of genomic compartmentalization.
Tanay A, Cavalli G.
Progressive polycomb assembly on H3K27me3 compartments generates polycomb bodies with developmentally regulated motion
Cheutin T, Cavalli G.
Polycomb: a paradigm for genome organization from one to three dimensions.
Delest A, Sexton T, Cavalli G.
Three-Dimensional Folding and Functional Organization Principles of the Drosophila Genome.
Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, Parrinello H, Tanay A, Cavalli G.
Molecular biology. EZH2 goes solo
Cavalli, G.
Trithorax group proteins: switching genes on and keeping them active.
Schuettengruber B, Martinez AM, Iovino N, Cavalli G.
Rolling ES Cells Down the Waddington Landscape with Oct4 and Sox2
Iovino, N., Cavalli, G.
Polycomb group proteins: repression in 3D.
Bantignies F, Cavalli G.
Editorial Review
Hetzer, M., Cavalli, G
Chromatin folding : from linear chromosomes to the 4D nucleus.
Cheutin, T., Bantignies, F., Leblanc, B., Cavalli, G.
From linear genes to Epigenetic inheritance of three dimensional Epigenomes.
Cavalli, G.
A chromatin insulator driving three-dimensional Polycomb response element (PRE) contacts and Polycomb association with the chromatin fiber.
Comet, I., Schuettengruber, B., Sexton, T., Cavalli, G
Polycomb-dependent Regulatory Contacts between Distant Hox Loci in Drosophila,
Bantignies, F., Roure, V., Comet, I., Leblanc, B., Schuttengruber, B., Bonnet, J., Tixier, V., Mas, A., Cavalli, G.
The DUBle life of polycomb complexes
Schuettengruber, B., Cavalli, G
Uncovering a tumor-suppressor function for Drosophila polycomb group genes.
Martinez, AM., Cavalli, G
Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice.
Schuettengruber B, Cavalli G.
Genomic interactions: Chromatin loops and gene meeting points in transcriptional regulation
Sexton, T., Bantignies, F., Cavalli, G.
Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling
Martinez, AM., Schuettengruber, B., Sakr, S., Janic, A., Gonzalez, C., and Cavalli, G.
Functional Anatomy of Polycomb and Trithorax Chromatin Landscapes in Drosophila Embryos
Schuettengruber B, Ganapathi M, Leblanc B, Portoso M, Jaschek R, Tolhuis B, van Lohuizen M, Tanay A, Cavalli G.
Epigenetics and the control of multicellularity. Workshop on Chromatin at the Nexus of Cell Division and Differentiation.
Reuter G, Cavalli G.
Chapter 2 polycomb group proteins and long-range gene regulation.
Mateos-Langerak J, Cavalli G
The Role of RNAi and Noncoding RNAs in Polycomb Mediated Control of Gene Expression and Genomic Programming.
Portoso, M, and Cavalli, G.
Idefix insulator activity can be modulated by nearby regulatory elements.
Brasset E, Bantignies F, Court F, Cheresiz S, Conte C, Vaury C.
Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex.
Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V.
Visualizing macromolecules with fluoronanogold: from photon microscopy to electron tomography.
Cheutin, T., Sauvage, C., Tchélidzé, P., O
Chromosome kissing
Cavalli, G.
Genome Regulation by Polycomb and Trithorax Proteins.
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G.
Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions.
Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T
Polycomb controls the cell fate.
Negre N, Cavalli G.
Mapping the distribution of chromatin proteins by ChIP on chip.
Negre N, Lavrov S, Hennetin J, Bellis M, Cavalli G.
From genetics to epigenetics: the tale of Polycomb group and trithorax group genes.
Grimaud C, Negre N, Cavalli G.
PRE-Mediated Bypass of Two Su(Hw) Insulators Targets PcG Proteins to a Downstream Promoter.
Comet I, Savitskaya E, Schuettengruber B, Negre N, Lavrov S, Parshikov A, Juge F, Gracheva E, Georgiev P, Cavalli G.
Chromosomal Distribution of PcG Proteins during Drosophila Development.
Negre N, Hennetin J, Sun LV, Lavrov S, Bellis M, White KP, Cavalli G.
The role of polycomb group proteins in cell cycle regulation during development.
Martinez AM, Cavalli G.
Polycomb group-dependent Cyclin A repression in Drosophila.
Martinez AM, Colomb S, Dejardin J, Bantignies F, Cavalli G.
RNAi components are required for nuclear clustering of Polycomb group response elements.
Grimaud C, Bantignies F, Pal-Bhadra M, Ghana P, Bhadra U, Cavalli G.
Chromatin and epigenetics in development: blending cellular memory with cell fate plasticity.
Cavalli G.
Cellular memory and dynamic regulation of polycomb group proteins.
Bantignies F, Cavalli G.
Epigenetic inheritance of chromatin states mediated by Polycomb- and trithorax group proteins in Drosophila
Déjardin, J., and Cavalli, G.
Recruitment of Drosophila Polycomb Group proteins to chromatin by DSP1
Déjardin, J., Rappailles, A., Cuvier, O., Grimaud, C., Decoville, M., Locker, D., and Cavalli, G.
Recruitment of Drosophila Polycomb group proteins to chromatin by DSP1
Dejardin J, Cavalli G.
The epigenome network of excellence.
Akhtar A, Cavalli G
Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line
Aravin, A. A., Klenov, M. S., Vagin, V. V., Bantignies, F., Cavalli, G., and Gvozdev, V. A.
Chromatin inheritance upon Zeste-mediated Brahma recruitment at a minimal cellular memory module
Déjardin, J., and Cavalli, G.
Interaction between GAF and Mod(mdg4) proteins promotes insulator bypass in Drosophila
Melnikova, L., Juge, F., Gruzdeva, N., Mazur, A., Cavalli, G., and Georgiev, P.
Combined immunostaining and FISH analysis of polytene chromosomes
Lavrov, S., Déjardin, J., and Cavalli, G.
SNR1 is an essential subunit in a subset of drosophila brm complexes, targeting specific functions during development
Zraly, C. B., Marenda, D. R., Nanchal, R., Cavalli, G., Muchardt, C., and Dingwal, A.K.
Protein-DNA interaction mapping using genomic tiling path microarrays in Drosophila
Sun, L. V., Chen, L., Greil, F., Nègre, N., Li, T. R., Cavalli, G., Zhao, H., Van Steensel, B., and White, K.
Inheritance of Polycomb-dependent chromosomal interactions in Drosophila
Bantignies, F., Grimaud, C., Lavrov, S., Gabut, M., and Cavalli, G.
Chromatin as a eukaryotic template of genetic information
Cavalli, G.
The MYST Domain Acetyltransferase Chameau Functions in Epigenetic Mechanisms of Transcriptional Repression
Grienenberger, A., Miotto, B., Sagnier, T., Cavalli, G., Schramke, V., Geli, V., Mariol, M. C., Berenger, H., Graba, Y., and Pradel, J.
Epigenetic Inheritance of active chromatin after removal of the main transactivator
Cavalli, G., Paro, R.
Mapping DNA target sites of chromatin-associated proteins by formaldehyde cross-linking in Drosophila embryos.
Cavalli, G., Orlando, V., and Paro, R.
The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis
11) Cavalli, G., and Paro, R.
Thèses et hdr
Study of the three-dimensional folding of chromatin into topological domains 27/11/2019
Defended by Quentin Szabo on 27/11/2019 under the supervision of Frédéric Bantignies and Giacomo Cavalli
Dynamic role of PRC1 during normal development and tumorigenesis in Drosophila melanogaster 16/11/2018
Defended by Vincent Loubière on 16/11/2018 under the supervision of Anne/Marie Martinez and Giacomo Cavalli
Characterization of Polycomb group protein binding site diversity in Drosophila 29/09/2017
by Marianne Entrevan on 29/09/2017 under the supervision of Giacomo Cavalli and Bernd Schuttengruber
Stable transgenerational inheritance of alternative chromatin states in Drosophila melanogaster 16/12/2015
Defended by Filippo Ciabrelli on 16/12/2015 under the supervision of Giacomo Cavalli
Histone H3 Serine 28 is essential for efficient Polycomb/mediated gene repression in Drosophila 09/02/2015
Defended by Yuk Kwong Yung on 09/02/2015 under the supervision of Giacomo Cavalli
Dynamic and differential targeting of Polycomb complexes during Drosophila melanogaster development 30/11/2012
Anna Delest, 30/11/2012
Differential function of Polycomb group proteins during Drosophila development 24/11/2011
Soutenue par Samy Sakr le 24/10/2011
Genomic and structural study of chromatin in Drosophila 24/10/2011
Benjamin Leblanc, 23/06/2011
The cell nucleus and gene regulation by Polycomb group proteins 28/10/2010
Bernd Stadelmayer 28/10/2010
Role of Polycomb and trithorax group proteins in the nuclear organization of homeotic genes in Drosophila melanogaster 10/12/2008
Virginie Roure 10/12/2008
Molecular studies of functional interactions between Polycomb group protein-dependent repression and gipsy insulators in Drosophila 20/11/2007
Itys Comet 20/11/2007
Search for Polycomb Group (PcG) Protein Target Genes in Drosophila 28/11/2005
Nicolas Nègre 28/11/2005
Sequences and factors required for epigenetic memory of chromatin states 27/05/2004
Jérôme Déjardin 27/05/2004